|
The 2024 ASCO Annual Meeting has a good startReprinted: Medicine Intelligence Network The abstract of the 2024 American Society of Clinical Oncology (ASCO) annual meeting was officially announced today. TROP2ADCsacituzumabtirumotecan (SKB264/MK-2870) jointly developed by MSD and Kolenbotai showed its eye-catching results in a phase III trial for the treatment of advanced triple negative breast cancer (TNBC), bringing a "good start" to the upcoming ASCO annual meeting this year. 1. What is the competitive landscape? TROP2, also known as Trophoblast Cell Surface Antigen 2, is a glycoprotein widely expressed in various tumors and almost non-existent in normal tissues. Among the transmembrane glycoproteins TROP1, 2, 3, and 4, only TROP2 has the ability to endow cancer cells with proliferation and invasion. At present, the physiological function of Trop-2 is not fully understood, but Trop-2 is associated with multiple intracellular axes, including the MAPK/PI3K/AKT pathway related to cancer cell proliferation, migration, and invasion. Trop-2 overexpression is associated with accelerated tumor growth and poor prognosis in breast cancer, gastric cancer, ovarian cancer and other cancers. This target is highly expressed in many solid tumors, such as glioma, cervical cancer, breast cancer, thyroid cancer, etc. And this also laid the groundwork for selecting indications for drug development in the later stage. As a hot star in the field of innovative drugs, ADC drugs use antibodies to precisely guide cancer cells and toxins to kill them. In addition, the bystander effect can cause damage to cancer cells that do not express the target antigen in the surrounding area through ADC. Trop-2 is an internalized cell membrane receptor that allows ADC drugs targeting it to enter intracellular lysosomes and release more cell permeable cytotoxic payloads, resulting in a stronger bystander effect. When it comes to the TROP-2ADC track, it is inevitable to mention the world's first TROP-2ADC to be launched - the Trodelvy. In April 2020, Trodelvy obtained FDA accelerated approval for second-line or above treatment of adult patients with metastatic triple negative breast cancer (mTNBC), becoming the world's first ADC approved for triple negative breast cancer. In 2021, the drug received full FDA approval and is currently on the market in over 30 countries including the United States, European Union, Australia, Canada, China, and Singapore. At present, it has three approved indications: second-line treatment of three negative breast cancer, second-line treatment of urothelial cancer, and multi line treatment of locally advanced or metastatic breast cancer. It has indeed lived up to expectations in terms of sales, with 2022 being its second full year on the market and sales reaching $680 million, a year-on-year increase of 79%. By 2023, its sales will have exceeded 1 billion US dollars. Analysts predict that Trodelvy's peak sales in the breast cancer field alone may approach $5 billion, and if it succeeds in other areas as well, sales may be even higher. And other MNCs are also accelerating their layout in this field, such as AstraZeneca following closely behind: in February of recent years, A
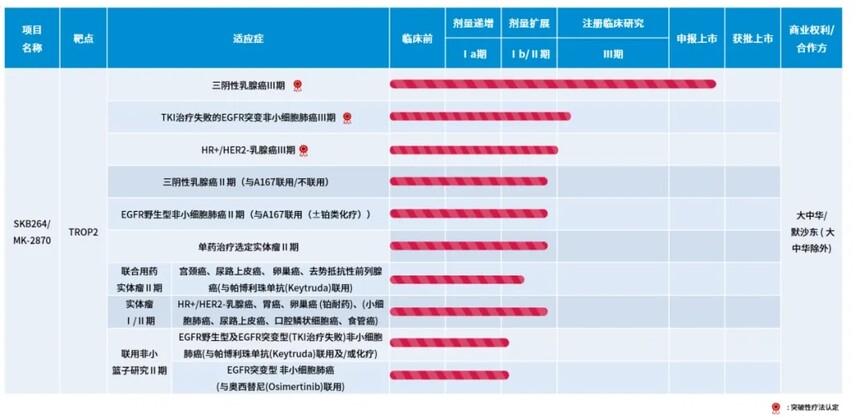
Figure 1: Research Status of SKB264 Image source: Kolombotai Company official website The sales peak of SKB264 is predicted by Southwest Securities to reach 3.7 billion yuan. Considering that SKB264 is the first class in China, the pipeline value can be estimated using five times the sales peak. Therefore, the value of this drug will reach 18.5 billion yuan! 3. Tumor drug feast The American Society of Oncology (ASCO) has always been a feast for anti-tumor drugs, and can be seen as a "showcase" for various pharmaceutical companies' oncology drugs, allowing everyone to appreciate their impressive clinical data. There are indeed many Chinese pharmaceutical companies worth paying attention to this year. The most representative is Xinda, which announced a month ago that it would release clinical data for approximately 20 drugs at ASCO this year. For example, IBI363 IBI389、IBI343、IBI310、 Taretinib and others. The Phase III clinical data of AK112 from Kangfang Biotechnology was also released today. Although it did not meet expectations, there are many reasons that do not affect its market launch, and it is already in the approved market period. MRG003 from Lepu Biotech has also been highly anticipated as an ADC drug with significant potential in the treatment of EGFR positive advanced solid tumors. Its mechanism of action is independent of the mechanisms that lead to EGFR-TKI and antibody targeted drug resistance, and based on innovative structural design of targeted combination chemotherapy, it is expected that MRG003 can overcome various types of drug resistance caused by common mutations. Preclinical studies have confirmed that MRG003 has significantly enhanced anti-tumor activity against T790M mutant NSCLC tumor models. Currently, according to the latest data presented orally at the ASCO conference, the ORR rate in phase I/II clinical trials for EGFR positive advanced solid tumor indications has reached 63%. 4. Conclusion This year's ASCO conference will undoubtedly have the presence of Chinese pharmaceutical companies, from Kelun to Lepu, from Xinda to Kangfang, Chinese pharmaceutical companies will surely have an increasing voice in the world of drug research and development, becoming pioneers in more and more fields! Reprinted link: Good start to the 2024 ASCO Annual Meeting _ Yaozhi News (yaozh. com) If there is any infringement, please contact us and we will delete it. |