|
Interpretation | KRAS inhibitors break through non pharmacological targets!Reprinted: Zhihu With the development of technology, there are increasingly more available drug targets for cancer. The target KRAS, which was previously considered untreatable, has also been approved for market and is currently a hot research and development topic in different mutants and indications. This article will start from the biological function and protein structure of KRAS, and explain the difficulties in drug development, research breakthroughs, and potential resistance mechanisms of KRAS. 1. The biological functions of RAS RAS-RAF-MEK-ERK is a classic signaling pathway primarily activated by growth factors, cytokines, and immune receptors. The RAS family (including KRAS, NRAS, and HRAS) is a key molecule that changes the structure of the "switch 1" and "switch 2" regions in proteins by switching between "On" (GTP loaded) and "Off" (GDP loaded) modes, thereby exerting its effects.
Any variation that affects the RAS-RAF-MEK-ERK pathway may abnormally activate this signaling pathway and form cancer, including mutations or amplifications in RTK, SHP2, NF1, RAS proteins, RAF family members, or MEK1/MEK2.
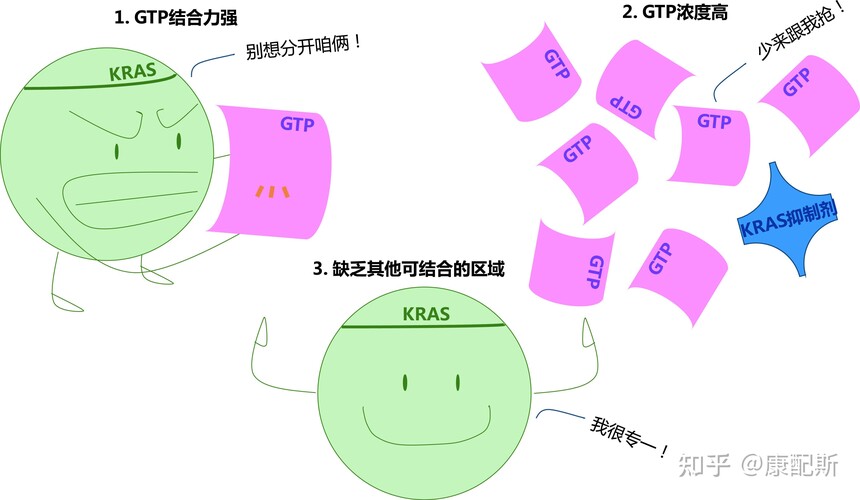
Nearly 95% of cancer-related RAS mutations are located at codons 12, 13, or 61, leading to a significant increase in the proportion of RAS-GTP: RAS-GDP and activating signaling pathways. KRAS is currently the RAS gene with high attention, and its G12C mutation has an incidence of about 14% in non-small cell lung cancer, while it is relatively rare in other types of cancer. Although KRAS is exclusive to other oncogenes, it often co occurs with tumor suppressor genes such as STK11, TP53, CDKN2A/CDKN2B, etc. In non-small cell lung cancer, when KRAS co mutates with tumor suppressor genes, it indicates poor efficacy of immune checkpoint inhibitors. 2. The Path of Drug Development for RAS The difficulty of targeting KRAS and other RAS proteins in pharmaceuticals is mainly due to the following three factors: The GTP binding affinity of RAS protein is extremely high, reaching the level of picomoles 2. The concentration of GTP inside the cell is too high, about 500nM 3. RAS protein lacks pocket regions for small molecule binding
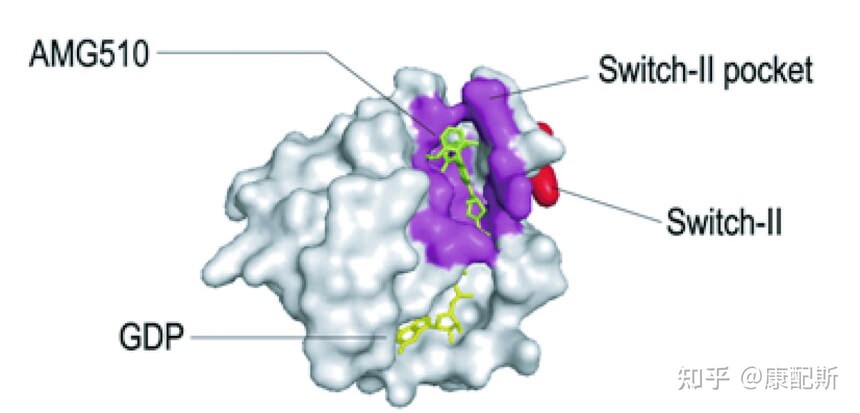
In addition, due to the different GTP binding sites of different KRAS mutants, it further increases the difficulty of designing KRAS inhibitors. In 2021, the first KRAS inhibitor Sotorasib (AMG 510) was approved for the treatment of non-small cell lung cancer with KRAS G12C mutation. Adagrasib (MRTX849) has also been approved for the same indication and extends the use of KRAS inhibitors to colorectal cancer. The indication is to use it in combination with cetuximab for colorectal cancer with KRAS G12C mutation. The above two drugs are defined as first generation KRAS inhibitors, which also target KRAS off (inactive state, binding to GDP) KRAS mutants. And new research is already attempting to develop KRAS mutants targeting "KRAS on" (activated state, binding to GTP), such as RM-018. Further pan KRAS inhibitors are expected to extensively target different KRAS mutants, amplify wild-type KRAS, and develop acquired resistance to KRAS inhibitors. Recently, Nature reported the discovery of a non covalent inhibitor that selectively binds to inactive KRAS, effectively preventing the activation of wild-type KRAS and a range of KRAS mutants. 3. Drug resistance mechanism and solution strategies The resistance mechanism of KRAS inhibitors still needs further exploration. According to theoretical speculation, potential resistance mechanisms include: Tumor mutation heterogeneity The occurrence of co mutations (especially at the binding sites of Sotorasib and Adagrasib, such as amino acid residues 12, 68, 95, and 96) Adaptive resistance (i.e. reactivation of the RAS-MAPK signaling pathway to a certain extent) Epithelial mesenchymal transition (EMT) Adenosquamous cell carcinoma transformation It is interesting that cell experiments have found that Sotorasib and Adagrasib both have some resistance mutations that make them sensitive to another drug, suggesting that although the two drugs are similar, they are not entirely the same. Potential solutions to address the aforementioned drug resistance issues include: Combination EGFR inhibitor (prevents activation of upstream EGFR) Combined SHP2 inhibitor (SHP2 is a key regulator upstream of the RAS-MAPK signaling pathway) Indirectly targeting RAS by inhibiting SOS1 (a key factor activating KRAS) Combined MEK inhibitor (downstream factor of RAS) Other: Inhibition of mTOR, CDK4/6, AURKA/AURKB/AURKC, WEE1, etc 4. Summary Although there have been some breakthroughs in the development of KRAS drugs at present, the research and development of KRAS inhibitors is still in its early stages. The development of pan KRAS inhibitors, the expansion of indications, and the exploration of resistance mechanisms still require extensive research to benefit more patients. reference: 1. Salman R Punekar, et al. The current state of art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol. 2022 Oct;19(10):637-655. 2. Chunxiao Zhu, et al. Targeting KRAS mutant cancers: from druggable therapy to drug resistance. Mol Cancer. 2022 Aug 4;21(1):159. 3. Dongsung Kim, et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023 Jul;619(7968):160-166. Reprinting link: Interpretation | KRAS inhibitors, breaking through non pharmacological targets- Zhihu (zhihu. com) If there is any infringement, please contact us and we will delete it. |