|
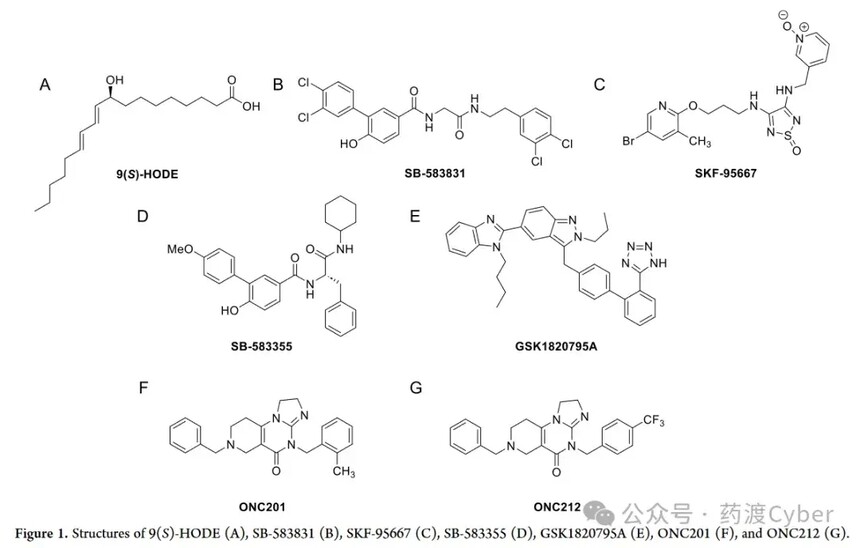
Discovery and optimization of potent and novel GPR132 (G2A) agonists by drug crossing Cyber analysisDiscovery and optimization of potent and novel GPR132 (G2A) agonists by drug crossing Cyber analysis Reprinted: Zhihu G protein coupled receptor GPR132, also known as G2A. In 1998, Ludwig et al. proposed the concept of proton sensitive GPCRs in 2003 and classified four orphan receptors of the AGPCR class: GPR4, TDAG8 (GPR65), OGR1 (GPR68), and G2A into this family. The role of proton sensing GPCRs was initially believed to be sensing tissue damage, inflammation, or tumor growth caused by a decrease in local pH. However, the proton sensitivity of G2A is controversial in the literature. Under physiological conditions, GPR4, OGR1, and G2A are commonly expressed, with G2A showing the strongest expression in white blood cells, neutrophils, and macrophages. G2A is expressed in peripheral sensory neurons co expressing transient receptor potential vanillic acid 1 channel (TRPV1). In this environment, G2A is closely associated with development through protein kinase C (PKC) activation and subsequent TRPV1 sensitization, and oxaliplatin induced neuropathic pain (OINP) persists. Therefore, inhibiting G2A is a new method to weaken OINP. G2A is also a factor promoting breast cancer metastasis. Because G2A is transcriptionally inhibited when peroxisome proliferator activated receptor γ is activated, its inhibition may contribute to the anti-tumor effect of rosiglitazone, and suggests that inhibiting G2A can be a new strategy for the treatment of breast cancer. G2A is also considered a lipid receptor, with lysophosphatidylcholine (LPC) being the first activator. However, the role of LPC as a G2A agonist is not yet clear. A study on LPC activation of G2A has been withdrawn, and several other studies have confirmed that LPC lacks G2A activation. One study even concluded that LPC is actually a weak antagonist of G2A (IC50>10 μ M). Oxidized lipids derived from linoleic acid (hydroxy octadecadienoic acid) or G2A ligands derived from arachidonic acid (hydroxy eicosaenoic acid, HETE) exhibit weak to moderate G2A activation, with linoleic acid metabolite 9-HODE considered the most effective endogenous G2A activator (Figure 1A). It is worth noting that the structure of G2A and its endogenous agonist form a complex 9-HODE, which was recently analyzed using cryo electron microscopy. Natural bacterial products such as Commentdamide, N-palmitoylglycine, and N-oleoyl glycine have also been found to act as G2A activators, reinforcing the concept that G2A is a signaling lipid receptor rather than a proton sensor. Compounds SB583831 (Figure 1B) and SKF-95667 (Figure 1C) are effective synthetic agonists, with EC50 values in the nanomolar range for SB-583831. On the other hand, compound SB-583355 (Figure 1D) and telmisartan analogue GSK1820795A (Figure 1E) are effective G2A antagonists.
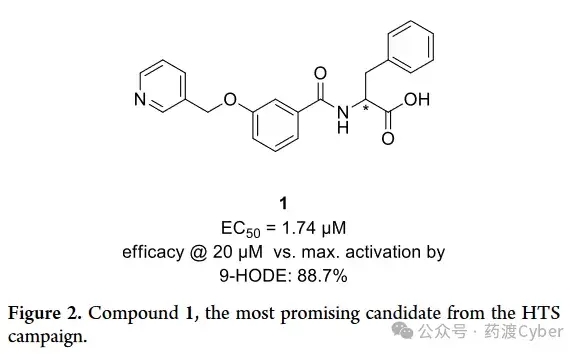
G2A activation has potential in hematopoiesis, sepsis, and acute myeloid leukemia (AML). The observation of anticancer drugs ONC201 and ONC212 (Figure 1F, G) activating G2A during β - inhibitory protein recruitment supports their anti AML activity. ONC212 also plays a significant pro apoptotic role in AML, but not in healthy bone marrow cells, and reduces the growth of AML in vivo, indicating that G2A activation may be a potential treatment for AML. In summary, current evidence suggests the potential of G2A activation and inhibition for the development of new therapies. This article introduces the discovery and SAR research of a novel effective and selective G2A agonist skeleton. The optimized analog outperforms the endogenous agonist 9-HODE in terms of G2A activation efficacy, and has excellent solubility and metabolic stability, making it a compound tool for studying G2A. 1. High throughput screening of G2A agonists In order to discover novel G2A agonists, 25000 compounds from the Enamine library were screened in a high-throughput screening (HTS) setting. The compounds are uniformly derived from all subsets of the library, and priority is given to library plates containing a larger number of carboxylic acid containing compounds, resulting in approximately 3000 carboxylic acids in the screening group. These compounds were tested at 50 μ M in functional cell-based assays to detect the accumulation of inositol monophosphate (IP-1) as a marker for human G2A activation. Compare efficacy with endogenous agonist 9-HODE (EC50=7.5 μ M). Select Hit (1, Figure 2) with the most promising efficacy (EC50=1.74 μ M) and synthetic accessibility for further optimization.
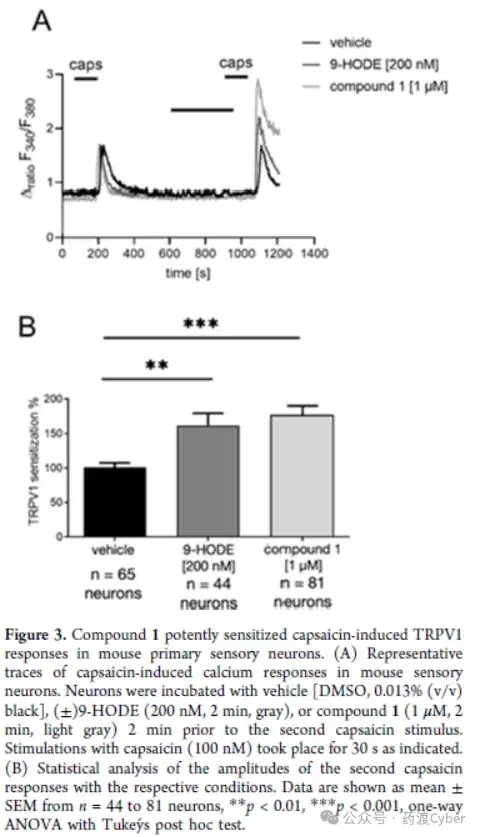
2. Hit validation of primary sensory neurons in mice To verify the effectiveness, compound 1 was tested using TRPV1 sensitization experiments based on calcium imaging. As mentioned earlier, the activation of G2A sensory neurons leads to enhanced TRPV1 activity in a Gq PKC dependent manner; Therefore, the author stimulated primary sensory neurons in mice twice with the same capsaicin stimulus (100nM, 30s) and compound 1 pre incubated before the second capsaicin stimulus. The analysis of the amplitude of calcium response [Δ ratio (F340/F380)] indicates that compound 1 can effectively be sensitive to capsaicin induced TRPV1 response, and is comparable to the reference G2A agonist 9-HODE (Figure 3A, B).
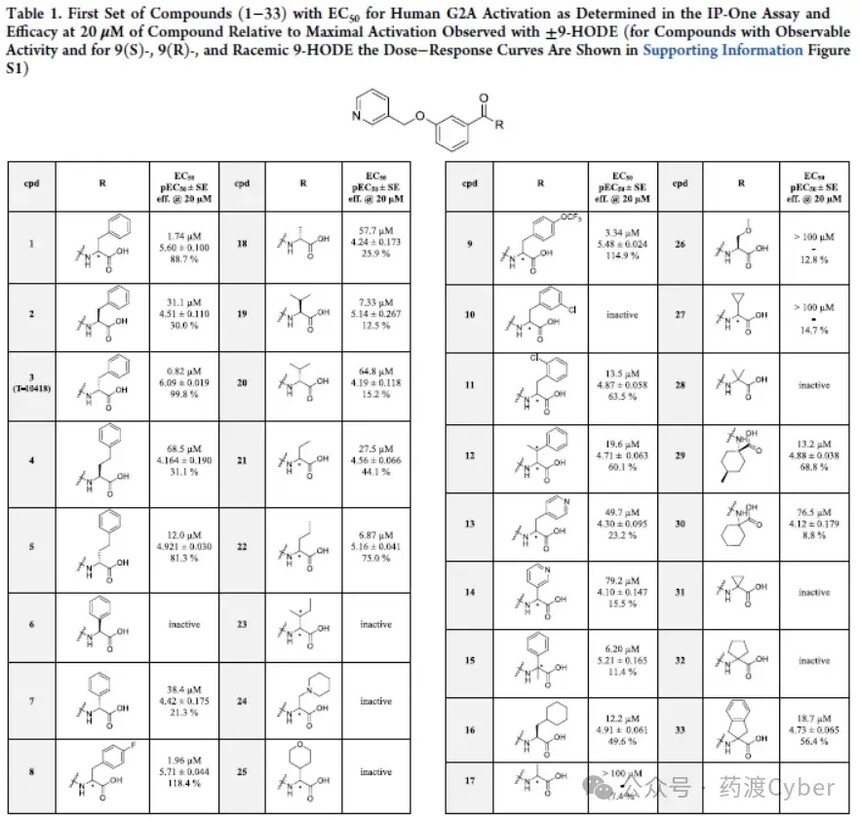
Firstly, two enantiomers of the original racemic product (1) from HTS were synthesized, and it was found that the (R) isomer (3 named T-10418, EC50=0.82 μ M) had an activity more than 30 times stronger than the (S) dimer (2, EC50=31.1 μ M). Compounds with high benzyl and phenyl residues (4-7) were studied, and their activity was significantly lower than Hit compounds with benzyl residues, but showed the same trend in stereochemistry. A small portion of aromatic substituents were tested in compounds 8-11. The results indicate that para substitution (8, EC50=1.96 μ M; 9, EC50=3.34 μ M) appears to have better tolerance than meta substitution (10, inactive) and ortho substitution (11, EC50=13.5 μ M). Compounds with pyridine rings (13 and 14) also showed only weak potency. Methyl substituents at the benzyl or carboxyl alpha positions are tolerable (12 and 15), but do not enhance activity. Next, a series of aliphatic substituents (16-32) with or without heteroatoms were analyzed. Generally speaking, except for compounds 19 (EC50=7.33 μ M) and 22 (EC50=6.87 μ M), the compounds in this series have weak activity (EC50>10 μ M) or no activity. If compound 19 is in the (S) - configuration, the corresponding enantiomer (R) has lower activity (20, EC50=64.8 μ M). However, compound 19 exhibits very limited efficacy at>20 μ M, which is significantly inferior to enantiomer (R), consistent with previous observations of the (R) - configuration. Finally, compound 33 with dihydroindene substitution only exhibited slight activity.
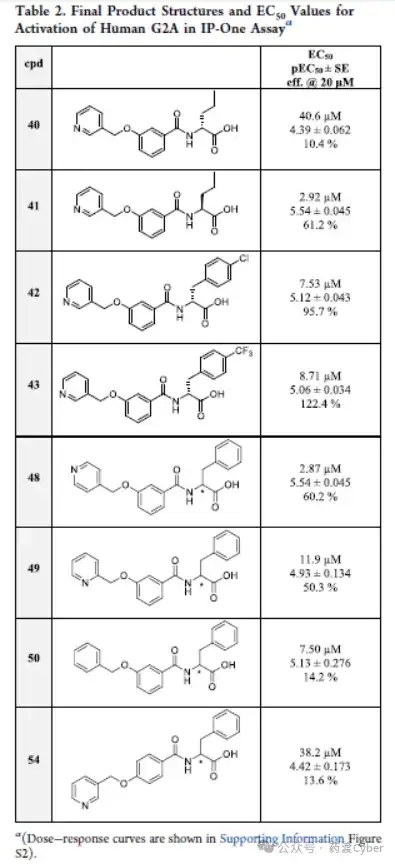
Considering the SAR results of the first batch of compounds (2-33), a second batch of compounds was designed and synthesized. The G2A activity of the second batch of compounds is shown in Table 2.
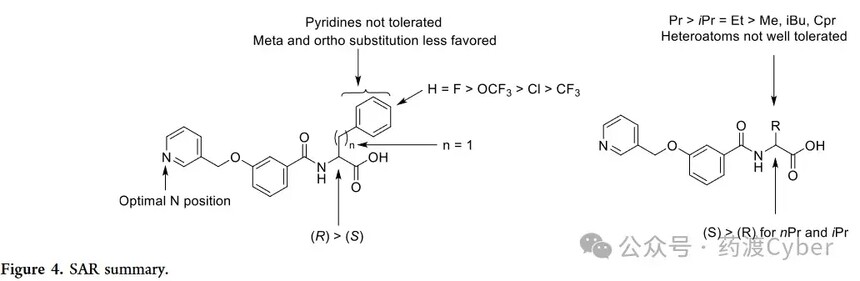
From the previous series, racemic compound 22 is the most promising derivative among aliphatic substituted benzene ring derivatives. Afterwards, its enantiomers were synthesized and tested. In this case, as with compounds 19 and 20, the (S) - enantiomer (41) with EC50=2.92 μ M exhibited higher potency than its (R) - enantiomer (40, EC50=40.6 μ M). Compounds 42 and 43 are both in the R configuration and have para substituents on the benzene ring on the right side of the molecule. As shown in the previous group of compounds, these para substituents are tolerable, but they did not lead to an improvement in EC50, although 43 exhibited the greatest activation effect. Next, racemic compounds with different nitrogen atom positions on the pyridine ring were studied. Compound 48 with adjacent nitrogen is effective, showing EC50=2.87 μ M, while compound 49 with adjacent nitrogen and compound 50 with benzene ring only have weak activity. Finally, compound 54 with a para configuration of substituents in the central ring results in loss of efficacy. The SAR results are shown in Figure 4.
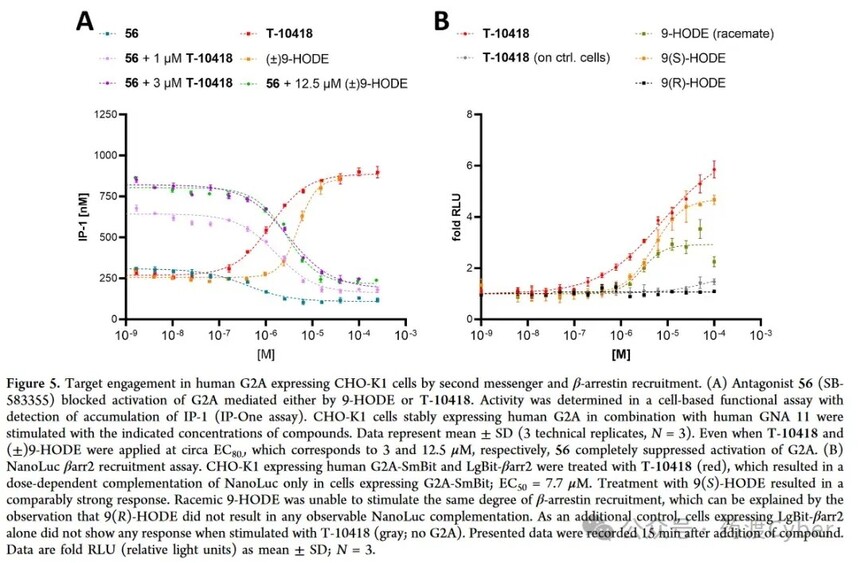
In order to use it as a reference tool compound, the antagonist SB-583355 (56) was also prepared. In the IP One assay, it was subsequently investigated whether the described G2A antagonist 56 (SB-583355) could counteract the activation of G2A by compound T-10418 (Figure 5A). Compound 56 completely inhibited the activation of T-10418 in the same dose-dependent manner as the reference agonist 9-HODE.
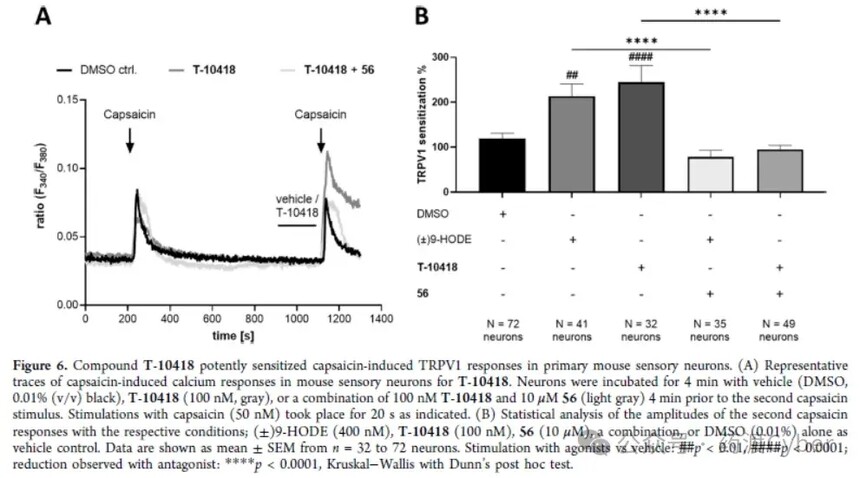
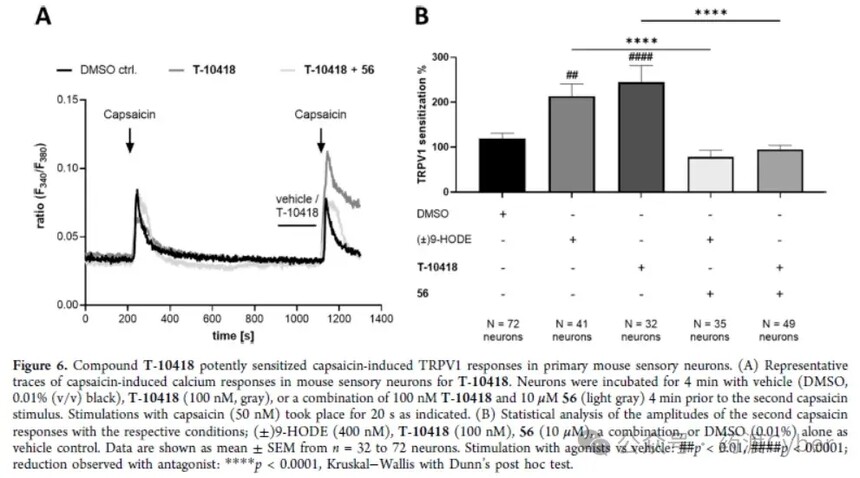
3. Directly recruit by combining G2A and β - Arestin-2 Direct binding of T-10418 to G2A in membrane proteins obtained from CHO-K1 cells stably expressing human G2A was analyzed by LC-MS. The membrane protein from wild-type CHO-K1 was used as a control. Although the affinity of T-10418 may be in the nM range, there is a small but statistically significant difference between the membrane proteins of transfected and untransfected cells at higher concentrations of T-10418 (600 nM as shown in Supporting Information Figure S3), which directly interacts with G2A. In order to further characterize T-10418 as a G2A ligand, the authors utilized the split luciferase complementation assay report β - arrestin2 [β arr2; uniport ID P32121] to recruit to G2A after receptor activation, confirming the direct interaction of the compound with the target in cells (Figure 5B). 4. Directly recruit by combining G2A and β - Arestin-2 Direct binding of T-10418 to G2A in membrane proteins obtained from CHO-K1 cells stably expressing human G2A was analyzed by LC-MS. The membrane protein from wild-type CHO-K1 was used as a control. Although the affinity of T-10418 may be in the nM range, there is a small but statistically significant difference between the membrane proteins of transfected and untransfected cells at higher concentrations of T-10418 (600 nM as shown in Supporting Information Figure S3), which directly interacts with G2A. In order to further characterize T-10418 as a G2A ligand, the authors utilized the split luciferase complementation assay report β - arrestin2 [β arr2; uniport ID P32121] to recruit to G2A after receptor activation, confirming the direct interaction of the compound with the target in cells (Figure 5B). 5. Target function of sensory neurons in mice T-10418 was tested in the TRPV1 sensitization assay of mouse sensory neurons; Figure 6 shows that 100nM T-10418 resulted in an average of 244% TRPV1 sensitization, equivalent to or exceeding the sensitization observed after treatment with 400nM 9-HODE. Therefore, T-10418 is more active than racemic compound 1, and the sensitization effect of TRPV1 caused by 1 μ M is comparable to the results observed using 200nM (±) 9-HODE (Figure 3). In addition, the TRPV1 sensitization effect of the two agonists T-10418 and 9-HODE was completely eliminated after co treatment with G2A antagonist 56, providing additional evidence for the interaction between T-10418 and the target.
Discovery and optimization of potent and novel GPR132 (G2A) agonists by drug crossing Cyber analysis Reprinted: Zhihu G protein coupled receptor GPR132, also known as G2A. In 1998, Ludwig et al. proposed the concept of proton sensitive GPCRs in 2003 and classified four orphan receptors of the AGPCR class: GPR4, TDAG8 (GPR65), OGR1 (GPR68), and G2A into this family. The role of proton sensing GPCRs was initially believed to be sensing tissue damage, inflammation, or tumor growth caused by a decrease in local pH. However, the proton sensitivity of G2A is controversial in the literature. Under physiological conditions, GPR4, OGR1, and G2A are commonly expressed, with G2A showing the strongest expression in white blood cells, neutrophils, and macrophages. G2A is expressed in peripheral sensory neurons co expressing transient receptor potential vanillic acid 1 channel (TRPV1). In this environment, G2A is closely associated with development through protein kinase C (PKC) activation and subsequent TRPV1 sensitization, and oxaliplatin induced neuropathic pain (OINP) persists. Therefore, inhibiting G2A is a new method to weaken OINP. G2A is also a factor promoting breast cancer metastasis. Because G2A is transcriptionally inhibited when peroxisome proliferator activated receptor γ is activated, its inhibition may contribute to the anti-tumor effect of rosiglitazone, and suggests that inhibiting G2A can be a new strategy for the treatment of breast cancer. G2A is also considered a lipid receptor, with lysophosphatidylcholine (LPC) being the first activator. However, the role of LPC as a G2A agonist is not yet clear. A study on LPC activation of G2A has been withdrawn, and several other studies have confirmed that LPC lacks G2A activation. One study even concluded that LPC is actually a weak antagonist of G2A (IC50>10 μ M). Oxidized lipids derived from linoleic acid (hydroxy octadecadienoic acid) or G2A ligands derived from arachidonic acid (hydroxy eicosaenoic acid, HETE) exhibit weak to moderate G2A activation, with linoleic acid metabolite 9-HODE considered the most effective endogenous G2A activator (Figure 1A). It is worth noting that the structure of G2A and its endogenous agonist form a complex 9-HODE, which was recently analyzed using cryo electron microscopy. Natural bacterial products such as Commentdamide, N-palmitoylglycine, and N-oleoyl glycine have also been found to act as G2A activators, reinforcing the concept that G2A is a signaling lipid receptor rather than a proton sensor. Compounds SB583831 (Figure 1B) and SKF-95667 (Figure 1C) are effective synthetic agonists, with EC50 values in the nanomolar range for SB-583831. On the other hand, compound SB-583355 (Figure 1D) and telmisartan analogue GSK1820795A (Figure 1E) are effective G2A antagonists. 20240805-100733.jpg G2A activation in hematopoiesis and pus production
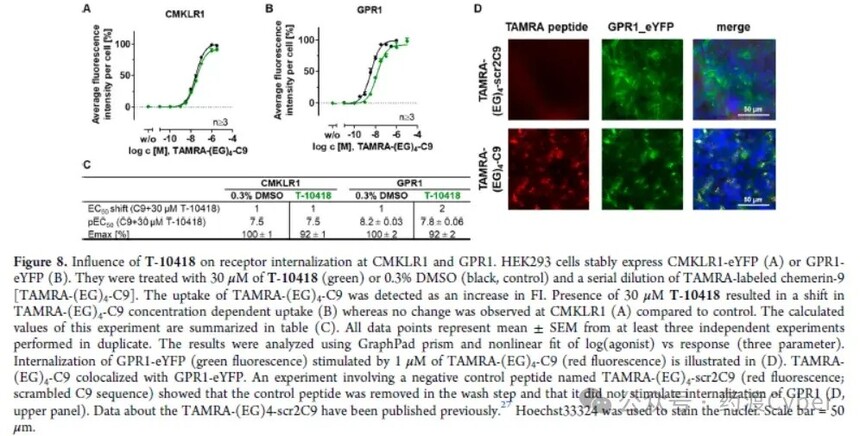
GPCR1(GPR1; Uniprot P46091 is activated by Camry (a 136 amino acid protein, also known as Camry-S157) and two other GPCRs in the Camry receptor family: chemokine like receptor 1 (CMKLR1) and CC motif chemokine receptor like 2 (CCRL2). The C-terminal nine peptide of Chemerin-S157, called Chemerin-9 (C9), has been identified to activate CMKLR126 and GPR1. For GPR1, only inhibitory protein recruitment can be detected, while for CMKLR1, G protein signaling transduction can also be detected after ligand stimulation. Therefore, the impact of T-10418 on internalization at GPR1 and CMKLR1 and β arr2 recruitment was determined. The binding of C9 with the fluorescent dye TAMRA (tetramethylrhodamine) can visualize its localization and cellular uptake. Treating cells expressing CMKLR1-eYFP or GPR1-eYFP with TAMRA - (EG) 4-C9 series dilutions resulted in an increase in TAMRA specific red fluorescence due to ligand binding and cellular uptake. Then co treated with 30 μ M T-10418. Although CMKLR1 was not affected by T-10418 (Figure 8A), the EC50 of HEK293 cells stably transfected with GPR1-eYFP showed a 2-fold change compared to the control without the compound, indicating that T-10418 had little effect on GPR1 internalization (Figures 8A, 8B, C). Figure 8D shows the internalization of GPR1 stimulated by TAMRA - (EG) 4-scr2C9 (disordered C9 sequence) compared to TAMRA - (EG) 4-C9 used as a negative control peptide.
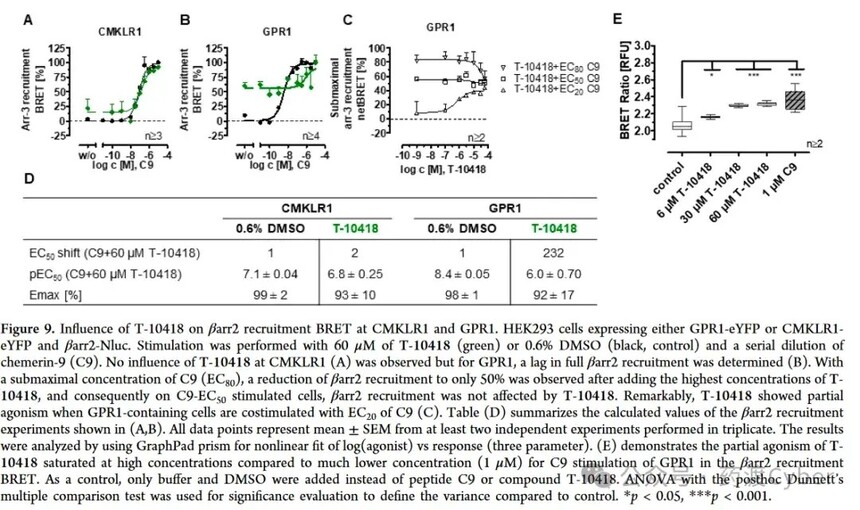
The regulation of T-10418 is stronger in the β arr2 recruitment bioluminescence resonance energy transfer (BRET) assay, which measures the association with β arr2 as a receptor ligand dependent activation reading (Figure 9). T-10418 only exhibited partial excitatory effects on GPR1 (Figure 9E), and this limited stimulation on β arr2 recruitment led to indirect testing of its effectiveness again. Compared with CMKLR1, T-10418 resulted in significant changes in C9 induced GPR1 activation (Figure 9A, B). Compared with the control without the compound, T-10418 at a concentration of 60 μ M weakened C9 stimulated β arr2 recruitment, and the EC50 of C9 changed by 232 times. When no endogenous ligand was applied to GPR1, the partial activation of 60 μ M T-10418 reached approximately 50% of the maximum β arr2 recruitment observed when C9 was fully activated (Figure 9B, w/o). In the control group, the maximal recruitment of inhibitory protein 3 and a half required 4nM C9. Finally, displacement binding assay 28 was performed using the recently developed method (Figure 10). Prove that T-10418 cannot replace the TAMRA labeled C9 bound to the Nluc-GPR1 construct, while C9 shows complete substitution with a Ki of 35.4 nM. Therefore, T-10418 does not interfere with the binding of C9 to GPR1 at all, or the compound is a low affinity binding (agonist) at the positive C9 site, and its affinity is too low to demonstrate its effect within this experimental setting and concentration range. Although significant stimulation of β - arr2 recruitment was observed in the β arr2 NanoBRET assay (Figure 9B, C), the T-10418-mediated C9 induced internalization inhibition of GPR1 (Figure 8B) was limited, consistent with the identification of GPR1. Presto Tango selectivity (Figure 7). However, in the NanoBRET β - arr2 recruitment assay, T-10418 counteracted the effect of C9 [>EC50 (C9); 9C], indicating that T-10418 vs C9 has a regulatory effect on β - arr2 recruitment. When T-10418 is used as a pharmacological tool, significant modulation of GPR1 is expected to occur only at ≥ 10 μ M. Further research on T-10418 and GPR1 related compounds will help to further characterize their modes of action.
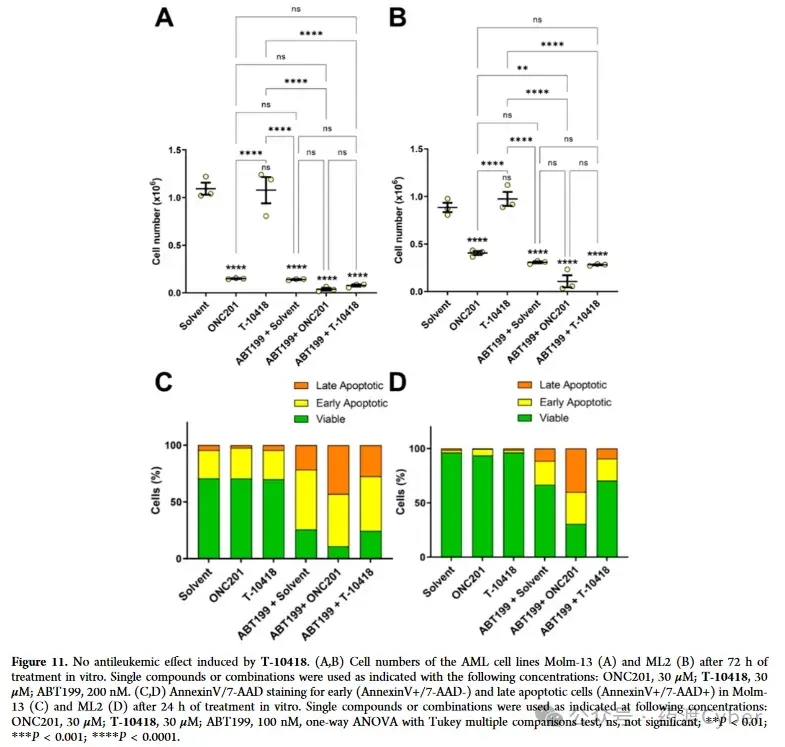
Encouraged by these observations, we continued to test the effects of T-10418 in combination with the prototype amiodarone ONC201 on AML cells. Incubate AML cell lines Molm-13 and ML-2 separately with ONC201 or T-10418 or in combination with Bcl2 inhibitor ABT-199 (known as Venetoclax). The synergistic effect between ONC201 and ABT-199. After 72 hours, it was observed/detected that the number of non surviving Molm-13 and ML-2 cells significantly decreased in the presence of ONC201, while T-10418 did not affect cell expansion or viability, as shown in Figure 11.
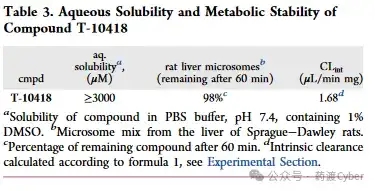
In addition, the author confirms that the combination therapy of ONC201 and ABT199 has a stronger effect on cell viability. The induction of cell apoptosis can be validated through AnnexinV/7-AAD measurement experiments using flow cytometry. The differential sensitivity of AML cells to ONC201 and T-10418, as well as the selectivity of T-10418 towards G2A, suggest that these cell lines may not respond to G2A activation, and ONC201 induces strong anti leukemia effects independent of G2A. Therefore, the author questioned whether these compounds activated the Gq pathway in AML cell lines and tested it in the IP One assay. To follow up on previous experiments, ONC201 and ONC212 were first tested on CHO-K1 cells expressing G2A+GNA11. Consistent with the report by Nii et al., ONC201 (EC50=38.6 μ M) exhibited limited activity and was lower than ONC212 (EC50=3.92 μ M). To test any response not mediated by receptors, a control experiment was conducted on CHO-K1 cells overexpressing only GNA1 but not G2A. When the concentration is ≥ 30 μ M, both ONC201 and ONC212 stimulate a significant increase in IP-1. This situation has not been observed before with 9-HODE, T-10418, or any derivatives listed in Tables 1 and 2. This indicates that ONC201 and ONC212 have addressed some other targets in CHO-K1 cells that can activate the Gq pathway. Then continue to test the response of Molm-13 and ML-2 cells in the IP One assay. After treatment with 9-HODE, T-10418, or ONC212 (more active on G2A), no activation of the Gq pathway was detected (Supporting Information Figure S5). Therefore, the strong effect of ONC201 in AML cells is likely independent of G2A, which may explain why T-10418 cannot inhibit AML cell survival. ONC201 and ABT199 have a significant additive effect, as reported by Nii et al. However, no additional inhibitory effect was detected when ABT-199 and T-10418 were used in combination. 7. Study on the cytotoxicity, water solubility, metabolic stability, and in vivo pharmacokinetics of T-10418 cells The toxicological characteristics of compound 1 (racemic) and T-10418 (enantiomer R) were tested in hepatocellular carcinoma cells (HepG2; DSMZ # ACC 180) using CellTiter Glo as the overall measurement method for metabolic activity and cell survival. The results were not affected after treatment with any compound at up to 100 μ M for 72 hours (threshold ≥ 90%) (Supporting Information Figure S6). To evaluate whether T-10418 is also suitable as a chemical tool for in vivo studies, its water solubility and metabolic stability were first studied, which are key factors for good drug absorption and low clearance rates. Determination of solubility in phosphate buffered saline (PBS) buffer solution
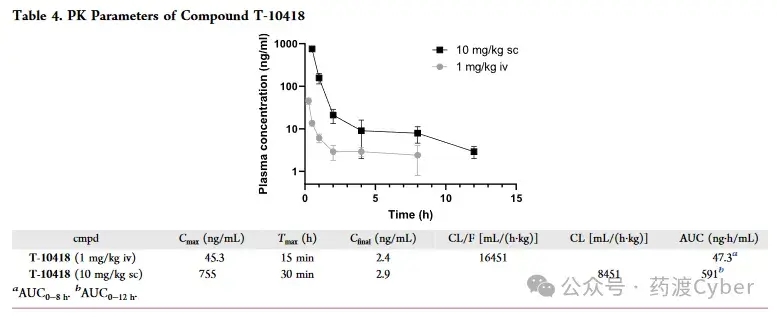
Due to its attractive properties such as high in vitro efficacy and excellent physicochemical properties, T-10418 was chosen for PK studies in male C57Bl/6N mice. Administer the compound intravenously at 1mg/kg (iv) and subcutaneously at 10mg/kg (sc) in PBS. The IV study measured plasma concentrations at 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours, while the subcutaneous study measured plasma concentrations at 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, and 12 hours. The obtained plasma concentration distribution and calculated PK parameters are shown in Table 4. The intravenous injection route produces the maximum plasma concentration after 15 minutes, and then the concentration decreases exponentially until the last measurement at 8 hours. On the other hand, when T-10418 was applied, the peak plasma concentration was reached at 30 minutes, making it the earliest recorded time point. Compared with intravenous injection, the AUC during subcutaneous injection is close to 10 times, which is fully consistent with the ratio between scanned intravenous injection doses. Overall, the compound exhibits high plasma concentration and good PK characteristics, making T-10418 a suitable candidate for in vivo studies of G2A agonist therapy potential.
8. Conclusion Orphan GPCR G2A is becoming an attractive target for various potential therapeutic applications, but it is still in the experimental stage to validate therapeutic efficacy through pharmacological G2A activation. A new G2 agonist skeleton was discovered and optimized in this article. T-10418 is one of the most effective G2A activators and exhibits high selectivity within the GPCR family. T-10418 failed to induce apoptosis in leukemia cell lines, indicating that G2A activation does not promote the anti leukemia effect of amiodarone. In addition, the excellent physicochemical and PK properties of T-10418 indicate its suitability as an in vivo compound tool. Therefore, T-10418 is a very valuable tool for studying the biology and therapeutic potential of G2A. Literature source Development of a Potent and Selective G2A (GPR132) Agonist | Journal of Medicinal Chemistry (acs.org) Reprinting link: Discovery and optimization of potent and novel GPR132 (G2A) agonists analyzed by Yaodu Cyber - Zhihu (zhihu. com) If there is any infringement, please contact us and we will delete it. |