|
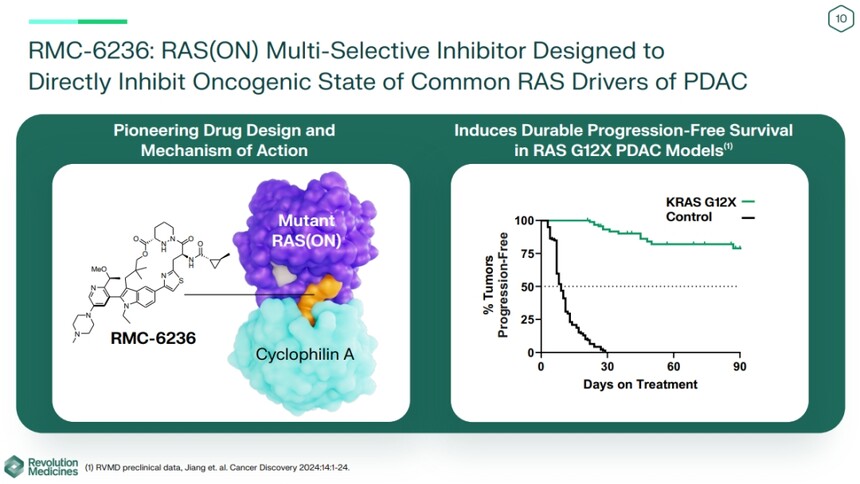
The pan RAS inhibitor has positive results in the treatment of pancreatic cancer in phase I, and will enter phase III clinicalThe pan RAS inhibitor has positive results in the treatment of pancreatic cancer in phase I, and will enter phase III clinical Reprinted: Medical Magic Cube On July 15, Revolution Medicines said in a report released to investors that its RAS (ON) multi selective inhibitor RMC-6236 would enter the phase III trial of pancreatic cancer after the encouraging results of phase I study. The latest results show that the tumor shrinkage rate of pancreatic ductal carcinoma (PDAC) patients is as high as 27%. RMC-6236 is an orally administered triple complex molecular gel that induces the formation of complexes between intracellular chaperone cycloserine protease A (CypA) and various RAS (ON) subtype mutants, including three oncogenic mutant proteins with the most common mutation sites (G12, G13, and Q61), thereby inhibiting tumor growth.
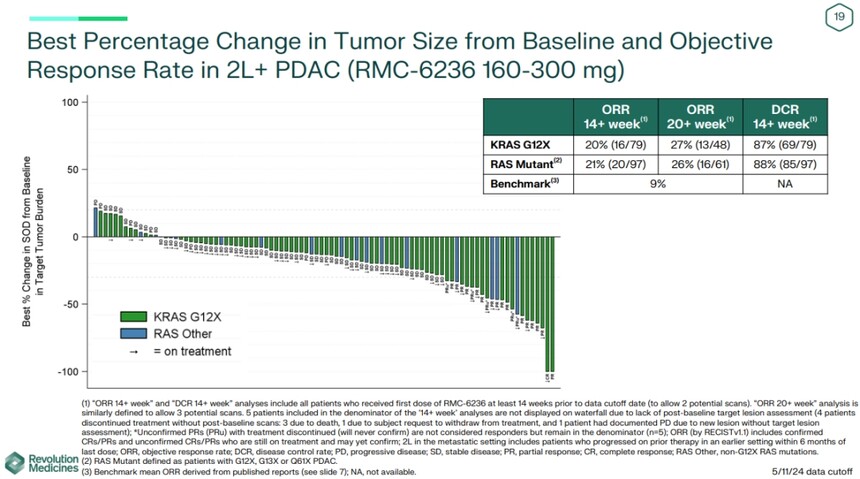
The latest results mainly target 127 PDAC patients, who took RMC-6236 doses ranging from 160mg to 300mg. In KRAS G12X mutation patients who have received one or more treatments in the past, the objective response rate (ORR) analyzed at 14 weeks or more is 20%, and the ORR analyzed at 20 weeks or more is 27%. For a wider population of RAS mutation patients (defined as G12X, G13X, or Q61X PDAC patients), the ORRs were 21% and 26%, respectively.
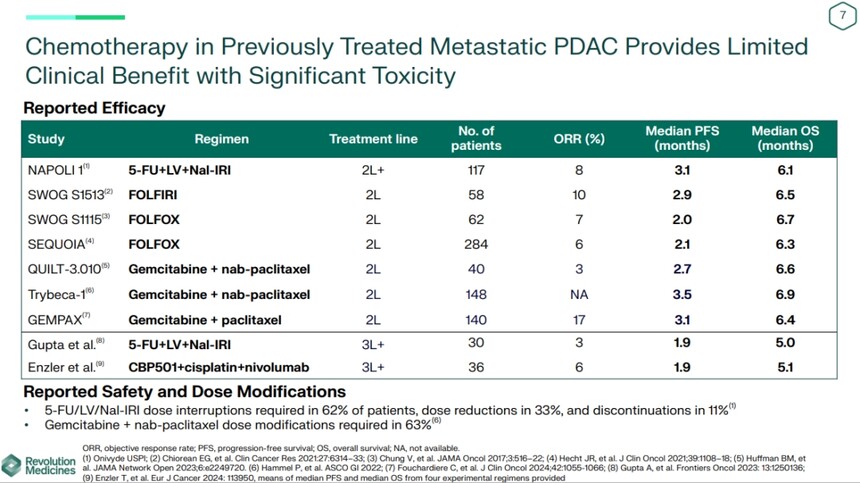
Revolution pointed out that, in contrast, chemotherapy, the standard treatment for second-line pancreatic cancer in clinical practice, has limited clinical benefits and significant toxicity, with an average ORR of about 9%.
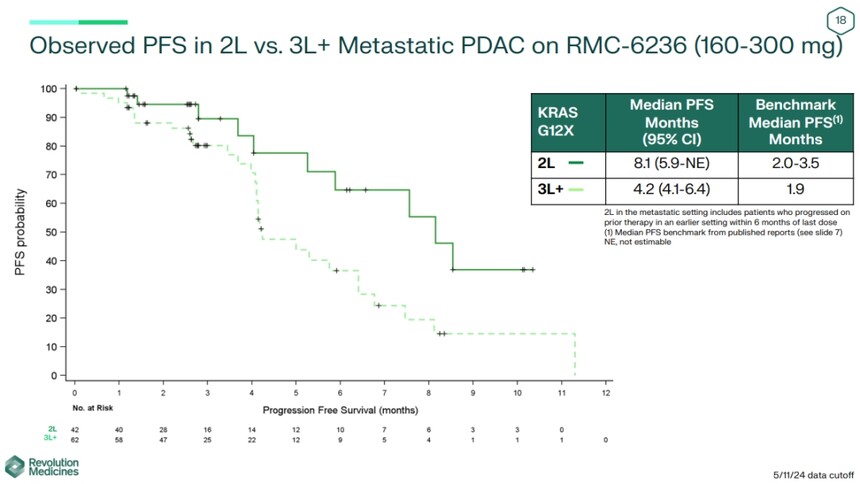
Meanwhile, the company stated that the median progression free survival (PFS) for second-line KRAS G12X patients reached 8.1 months, while the median PFS for third line and above patients was 4.2 months, and the median PFS for standard chemotherapy regimens is typically 2 to 3.5 months.
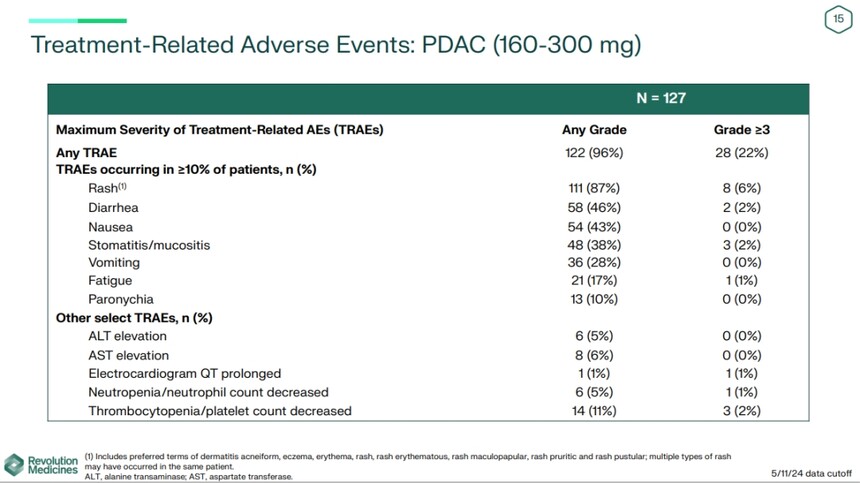
In terms of safety, the most common adverse reactions include rash (87%), diarrhea (46%), nausea (43%), stomatitis/mucositis (38%), and vomiting (28%) The incidence of grade 3 adverse reactions is 22%, and 28% of patients need to adjust their dosage, but there has been no discontinuation of medication.
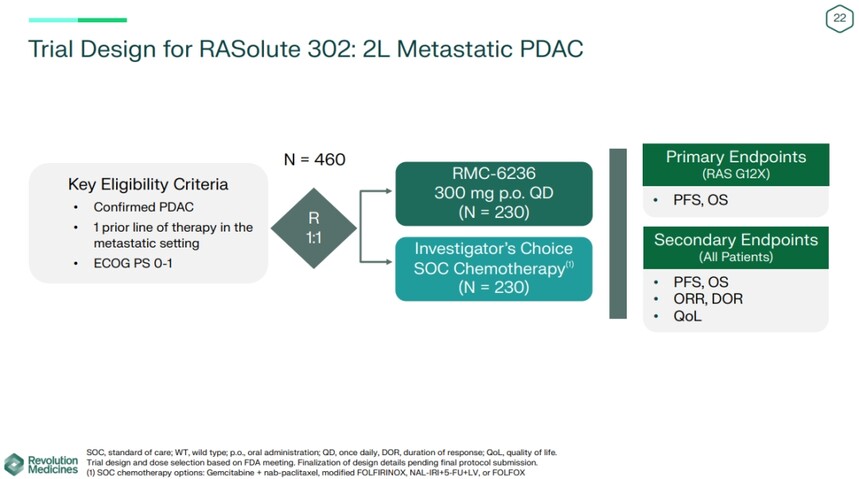
Revolution Company now plans to advance the 300mg dose of RMC-6236 to phase III clinical trials, comparing it with standard chemotherapy chosen by researchers in second-line patients with metastatic PDAC.
The RASolute 302 trial plans to recruit 460 patients and is expected to begin in the second half of the year. The primary endpoints of the study are PFS and overall survival (OS). PFS data is expected to be obtained in the first half of 2026. Reprint link: Pan RAS inhibitor treatment of pancreatic cancer phase I results are positive, and will enter phase III clinical medical news - ByDrug - one-stop medical resource sharing center - medical Rubik's Cube (pharmcube. com) If there is any infringement, please contact us and we will delete it. |