|
Nature Journal: Molecular Gel Development, Birth of a 'New Approach'Nature Journal: Molecular Gel Development, Birth of a 'New Approach' Reprinted: Medical Magic Cube Molecular glue is an important protein degrading agent that can achieve dual functions of recruiting target proteins and E3 ligases. Due to its small molecular weight, it has advantages in drug formation. However, currently most molecular gels are discovered by chance. The existing "rational design strategies" are mostly obtained through medicinal chemistry methods, optimizing the structure and drug properties of existing molecular gel compounds. Therefore, designing molecular gels targeting specific targets is currently a major challenge and a key problem that urgently needs to be overcome in the future.
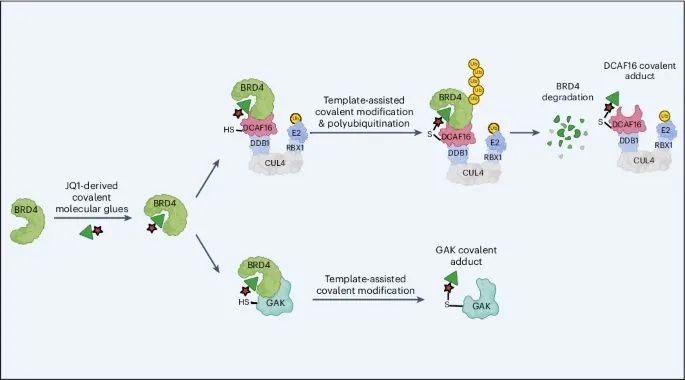
Recently, a paper was published in the journal Nature Chemical Biology, in which the authors discovered a molecular gel mechanism called "template assisted covalent modification" and developed a series of new BRD4 molecular gel degradation agents based on it. These molecular gels can recruit CUL4DCAF16 ligase to the second bromodomain of BRD4 (BRD4BD2). This study provides a new perspective for the design of covalent molecular adhesives and offers strategies for developing molecular adhesives with high potency and specificity.
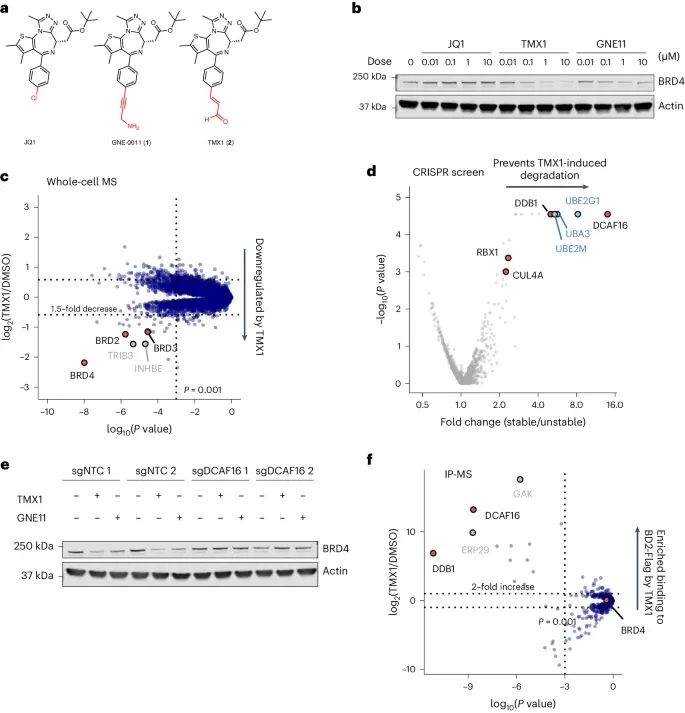
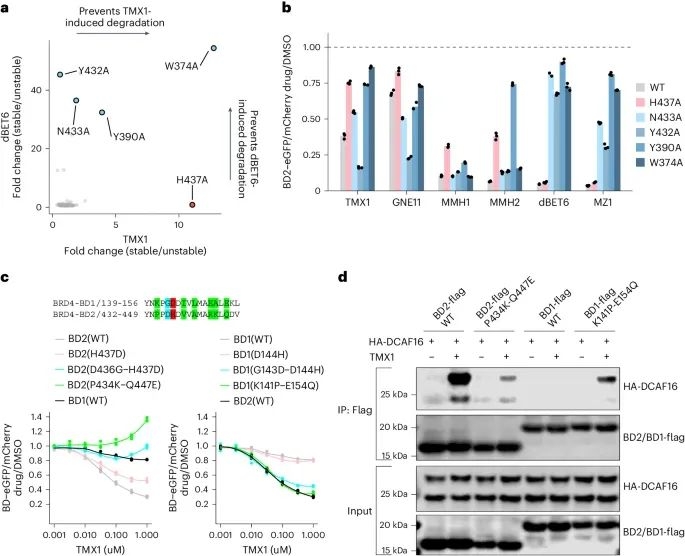
As shown in Figure 1a, the researchers first optimized the structures of JQ1 (a commercially available BRD4 selective inhibitor) and GNE-0011 (a preclinical BRD4 degrader) to obtain an acrolein analogue TMX1, which has a stronger degradation effect on BRD4 while maintaining selectivity for BRD4BD2. The study found that the main degradation target of TMX1 is BRD4, while its impact on the other two BET family proteins BRD2 and BRD3 is relatively small. In addition, mechanistic studies have found that TMX1 can degrade BRD4 by recruiting DCAF16.
Next, the researchers observed through time-resolved fluorescence energy transfer (TR-FRET) experiments that the interaction between DCAF16 induced by TMX1 and BRD4BD2 was more closely related than that between BRD4BD1. The experimental results indicate that TMX1 can selectively recruit DCAF16 to BRD4BD2, leading to the degradation of BRD4. In addition, at high concentrations, the interaction between TMX1 and DCAF16 decreases, which may be due to the formation of covalent bonds between TMX1 and DCAF16. Due to the presence of BRD4BD2 promoting covalent modification, researchers believe that this may be because it causes the acrolein warhead to be attacked by cysteine, hence naming this mechanism "template assisted covalent modification".
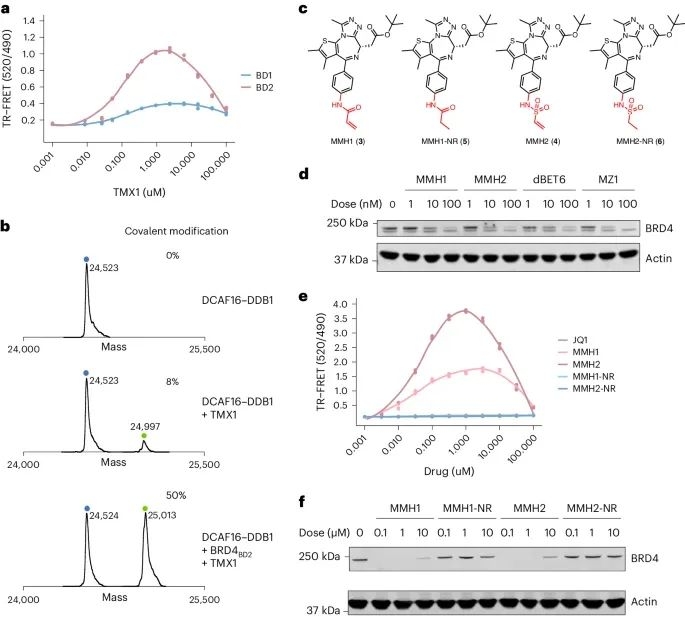
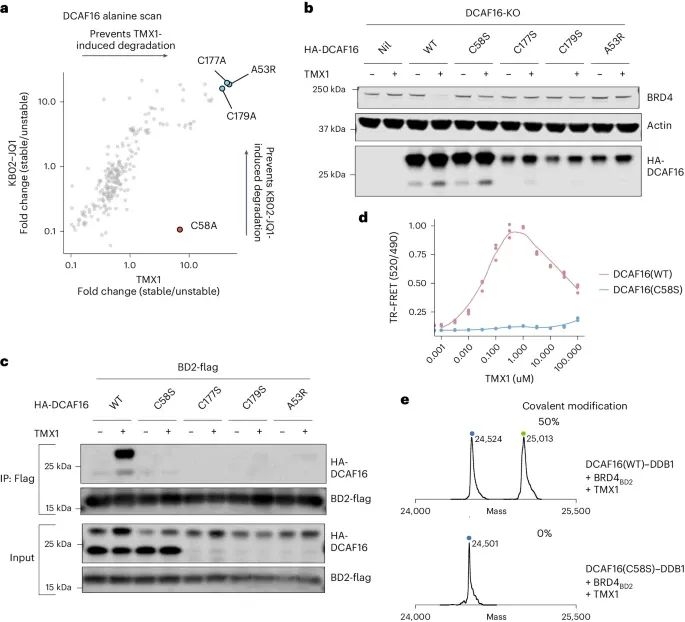
Then, the researchers synthesized two compounds with electrophilic warheads - acrylamide analogue MMH1 and vinylsulfonamide analogue MMH2 (Figure 2c). Compared with TMX1, MMH1 and MMH2 showed significant improvements in BRD4BD2 degradation activity and DCAF16 recruitment. In addition, MMH1 and MMH2 exhibit similar activity in the degradation of BRD4, and due to the covalent mechanism, they exhibit more persistent activity after elution. This indicates that the optimized warhead can enhance the effectiveness of the degradation agent and limit off target toxicity. Afterwards, the researchers characterized the structure of the ternary complex of BRD4BD2, DCAF16, and MMH2 using cryo electron microscopy (cryo EM). It was found that Cys58 on DCAF16 is a specific target for molecular gel degradation agents.
In addition, through systematic analysis of alanine mutations, researchers identified the amino acid residue His437 in BRD4BD2, which is crucial for the activity of molecular adhesive degradation agents.
Finally, the researchers explored whether "template assisted covalent modification" could be extended to other proteins beyond DCAF16, and found that GAK proteins could also interact with BRD4BD2 through this mechanism. In the presence of BRD4BD2, MMH2 and TMX1 significantly increased the covalent modification of GAK. Overall, researchers have elucidated a novel mechanism of covalent molecular glue interactions through a series of experiments - "template assisted covalent modification", providing new ideas for the rational design of covalent molecular glue. Note: The illustrations in the article are all from Nature Chemical Biology. reference material: Yen-Der Li et al. Template-assisted covalent modification underlies activity of covalent molecular glues. Nature Chemical Biology(2024) https://www.nature.com/articles/s41589-024-01668-4 Reproduction link: Nature sub journal: Molecular Gel Development, a "New Approach" Born Pharmaceutical News - ByDrug - One stop Medical Resource Sharing Center - Pharmcube.com If there is any infringement, please contact us and we will delete it. |